[ad_1]
In 1976 Alan Grodzinsky ’71, ScD ’74, was feeling a little frustrated.
He spent two years teaching a basic course in semiconductor physics and circuits in MIT’s Department of Electrical Engineering and Computer Science, learning materials in the fast-moving field as he progressed. This left him no time for research. Then a golden opportunity arose.
With the help of the late Irving London, founder of the Harvard-MIT Health Sciences and Technology Program, Grodzinsky earned a sabbatical at Boston Children’s Hospital under the mentorship of the late Mel Glimcher, chief of orthopedic surgery and a leading researcher in biology. human bones and collagen.
Glimcher wanted to start a research project on cartilage, the tough fiber matrix that covers joints, and osteoarthritis, the chronic, painful disease that breaks down this cartilage.
He was a perfect fit for Grodzinsky, 29, who earned his ScD by studying the electrical properties of collagen, one of the components of cartilage. At year’s end, he was on track ever since: trying to find effective treatments for osteoarthritis, the leading cause of chronic pain and disability worldwide. It affects more than 30 million Americans and hundreds of millions of people worldwide.
“It’s a huge financial and injury burden. “It certainly contributes to the loss of quality of life, if not fatal,” says Joseph Buckwalter, an orthopedic surgeon and osteoarthritis specialist based in Iowa, who has known Grodzinsky for decades. “The cost of total joint replacement, primarily the knee and hip, is one of our biggest healthcare expenses.”
No plans for the pain
The U.S. Food and Drug Administration has not approved any disease-modifying drugs—drugs that treat the underlying condition rather than just the symptoms—for osteoarthritis. What most patients can hope for is painkillers like Motrin, occasional steroid injections, and eventually joint replacement surgery, Grodzinsky says. More than one million knee and hip replacements are performed each year in the United States, and this number is expected to increase as the population ages.
While older people are most susceptible to osteoarthritis, Grodzinsky focused most of his research on younger people, particularly female athletes who often develop the condition after knee injuries.
Every year, tens of thousands of young women are injured in the anterior cruciate ligament of their knees. “When I teach a course on biomechanics at MIT,” Grodzinsky says, “I ask about ACL injuries, and as many hands are raised today as in the past. I recently taught a Harvard Medical School class, and four out of 20 students in my class suffered from ACL tears and one was in his third surgery.”
Doctors can fix these tears, he says, but both men and women with joint injuries are at risk of developing osteoarthritis in later years. While knee replacements can counteract the effects of osteoarthritis, doctors are reluctant to perform such surgeries on younger people because they will likely need to be repeated after the first artificial joint has worn off.
A knee implant can take years, Buckwalter says, but “I would have nightmares doing this to someone under 40 because the odds of needing another are almost overwhelming.”
Nanoparticle Rx
Grodzinsky says researchers have identified available drugs that can alleviate the onset of osteoarthritis, but the fact that cartilage does not have a natural blood supply hinders them. When doctors inject a steroid into the knee joint to reduce inflammation, the body removes most of the drug before it enters the cartilage.
To solve this problem, his lab has spearheaded research involving nanoparticles, human cadaver knees, and even missions to the International Space Station.
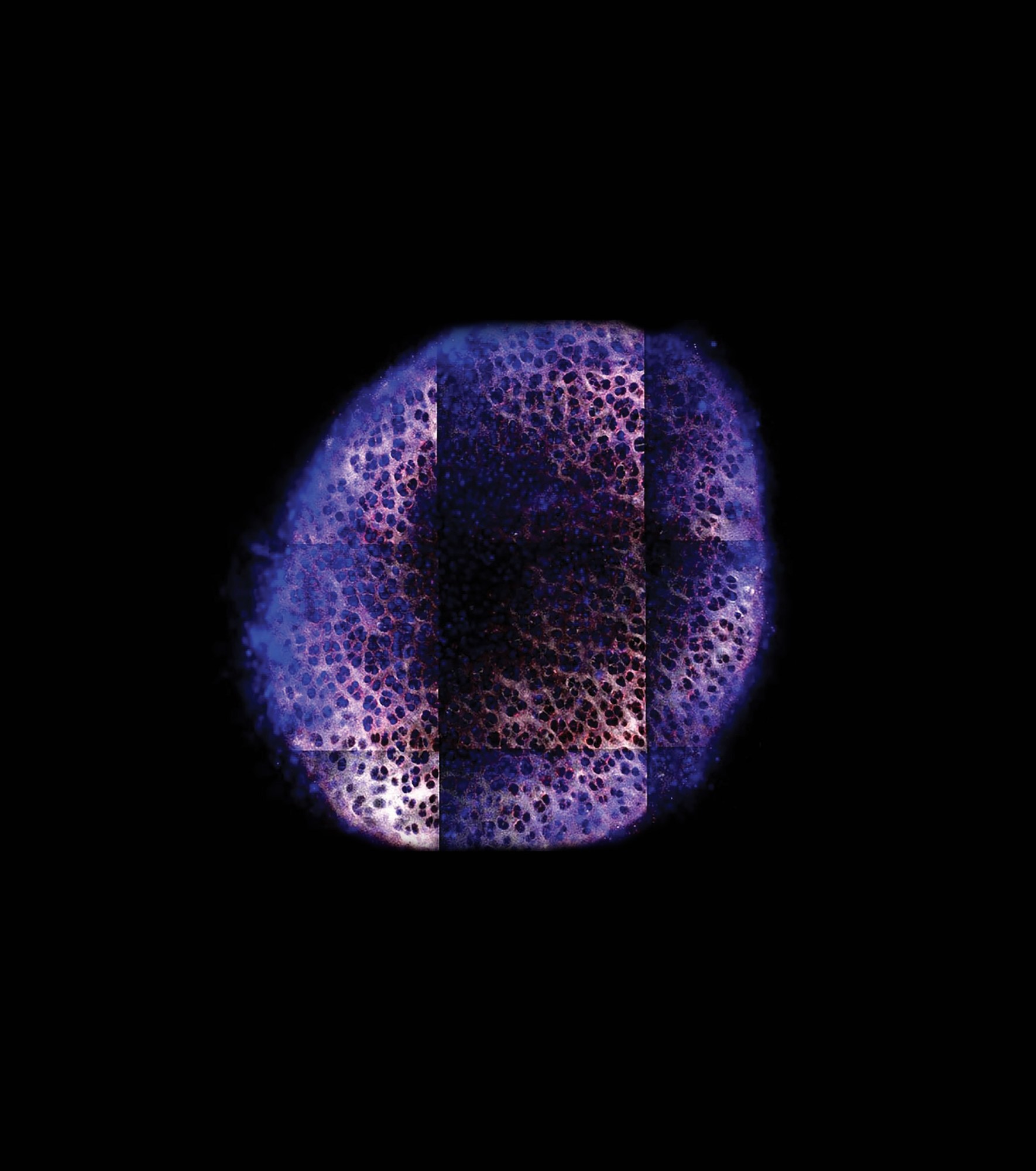
BRET GEIGER AND JEFF WYCKOFF
Starting with this leave over forty years ago, Grodzinsky learned a vital truth about cartilage. While the tissue fibers themselves provide some support for our joints, most of their strength comes from their electrostatic properties. “It turns out that about half of the compressive mechanical stiffness of our cartilage is due to electrostatic repulsive interactions between negatively charged sugar chains,” he says.
This negatively charged tissue matrix also provides a way to deliver drugs directly into tissue by loading them into positively charged nanoparticles. Grodzinsky’s team was able to show that such particles in human cadaver knee cartilage can prevent early inflammation and damage caused by injuries.
The first nanoparticle study was initiated a few years ago by Grodzinsky’s former doctoral student Ambika Bajpayee, MNG ’07, PhD ’15, now a professor at Northeastern University. Bajpayee then collaborated with Paula Hammond, head of MIT’s department of chemical engineering, who pioneered the use of nanoparticles to deliver drugs to cancerous tumors.
In the Grodzinsky lab, drug-containing nanoparticles are injected into the joints of animals, just like in human patients, and “after they go in, they can stay if used at the right concentration. for weeks”, embedded in the fibrous matrix.
The group focused on delivering two drugs already approved for human use.
One is the anti-inflammatory dexamethasone, which has been used successfully to treat respiratory problems in some hospitalized covid-19 patients. The other is insulin-like growth factor 1 (IGF-1), a hormone that promotes the growth of bone and cartilage tissue and is used in children born younger than normal.
Grodzinsky says dexamethasone reduces cartilage breakdown after an injury, while IGF-1 can promote tissue repair.
Animal studies have been done using IGF-1 in collaboration with Hammond, and Grodzinsky’s lab has also extended this experimental treatment to human tissues based on samples from dead humans. Garima Dwivedi, a postdoctoral researcher in the lab, says so far the lab has been able to obtain pieces of knee bone, cartilage and synovial joint capsule from 45 donors.
Dwivedi and colleagues put the samples into wells set on plastic plates and keep them metabolically active. They then apply a mechanical blow that mimics what happens with a knee injury. This releases inflammatory molecules known as cytokines and initiates a process similar to what happens in osteoarthritis.
Space
In this study, the researchers put the nanoparticles into culture medium that washes tissue samples – a technique they could also use in future experiments on the space station, which has become a magnet for researchers studying diseases of aging.
Scientists have known for years that human tissues age faster in low Earth orbit than on Earth, although the reasons are somewhat mysterious. One analysis estimated that astronauts’ muscles and bones atrophied 10 times faster in microgravity.
Figuring out how to repair joint damage could be crucial for future long-term space missions.
With funding from the NIH and NASA, Grodzinsky’s lab sent knee cartilage-bone plug and synovium tissue samples to the ISS in 2019 and 2020. They hoped to determine whether the osteoarthritis-like disease could be started “on a plate” to simulate what was happening. after a knee injury in humans – using the microgravity environment to explore and eliminate mechanical processes in the workplace – and try treating it with dexamethasone and IGF-1.
He says the initial results are encouraging. On the most recent trip to the ISS, the lab found that both drugs reduced damage to most of the cartilage samples.
“As most researchers these days emphasize that there will be no single magic bullet, we believe the ability to test drug combinations in vitro is an important step forward,” says Grodzinsky.
Dwivedi says the work in microgravity could also pay dividends for future space missions. He says astronauts who exercise intensely in space to counter the atrophy that muscles and bones undergo in weightless conditions are three times more likely to suffer impact injuries than people on Earth, so figuring out how to repair joint damage could be crucial. for future long-term space missions.
compassionate mentoring
Grodzinsky’s destiny always seemed to be to find a home at MIT.
Growing up on Long Island, where he attended public schools in the booming suburb of East Meadow, he occasionally visited his older brother, Stephen Grodzinsky ’65, SM ’67 at Burton House. “This looks great to me,” he remembers thinking.
He went on to receive his ScD from the late James Melcher, director of the school’s Electromagnetic and Electronic Systems Laboratory. A recession soon struck, however, and the only positions offered to him were a postdoctoral fellowship in icy Saskatchewan and an assistant professor of music and engineering in Brazil. His mentors, including Ioannis Yannas, best known for inventing faux leather, offered him a teaching position in electrical engineering, encouraging him to stay around. He has been at the Institute ever since.
In 1995, MIT established the Center for Biomedical Engineering to advance research, which was a new field at the time. Three years later, Grodzinsky was appointed to his current post as director. At that time, faculty membership changed to the newly formed Department of Biological Engineering, with joint assignments in EECS and mechanical engineering.
Grodzinsky believes that any research success he achieves is the direct result of “the tremendous doctoral students and postdoctoral students we have been able to achieve at MIT.” They, too, prospered under his compassionate mentorship.
“It was a pleasure working with him, primarily because he gives you so much independence to develop your own ideas,” says Postdoc Dwivedi. “And whoever you are and whatever stage of your career you are, he listens to you with great attention and respect.”

WEBB CHAMPION
He also appreciates his personal support. When his family in India contracted covid in April, he “gave me complete free time to take care of them,” he says.
At 74, Grodzinsky managed to avoid osteoarthritis, despite being in the primary risk category for the disease.
Maybe, she thinks, because her preoccupation as a musician has kept her fit. After years of piano lessons at the Third Street Music School Settlement in New York, he became principal violist of the MIT Symphony Orchestra as an undergraduate. He also played in a free string quartet after finishing his ScD and met his wife, Gail, while playing chamber music.
“For some reason, I just couldn’t find a way to leave,” she says, smiling after she officially set foot on campus as an 18-year-old student.
[ad_2]
Source link

